December 13th, 2016 saw history being made with the FDA making its first approval on a product which allows the cells from an individual’s knee to be grown on scaffolds and used for tissue engineering. The process called MACI, holds the power to dramatically improve the consequences of knee injuries for patients by taking healthy cartilage tissue and using it to repair defects and defective tissue.
How MACI Works
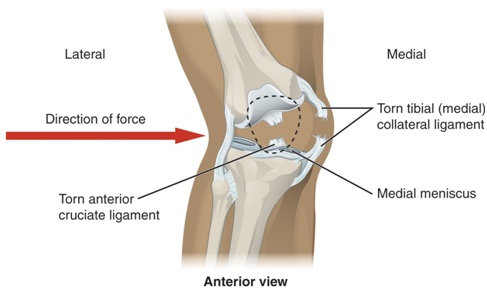
MACI (autologous cultured chondrocytes on porcine collagen membrane) is manufactured by the Massachusetts-based Vericel Corporation and formed from a patient’s autologous cells. Damaged tissue is treated by being removed and then replaced by an implanted bio-resorbable porcine-derived collagen membrane where the autologous cells have been expanded. The amount of MACI administered for implantation is determined by the size of the cartilage which needs repairing. MACI is available for adults and it can repair individual and full-thickness multiple cartilage defects of the knee. Due to the common occurrence of knee injuries in individuals, from causes such as muscle tears, weakness and fatigue, a variety of practices are needed with varying treatment plans for individuals.
The Impact
The treatment has been described by David Recker, MD, Vericel’s chief medical officer, as being, compared to microfracture, the alternative surgical treatment for cartilage repair commonly used, the first product to show a significant and statistically greater improvement in KOOS pain and function scores. It is the aim of Vericel, that MACI will enable a larger patient population to gain treatment for cartilage repair by giving orthopaedic surgeons an efficient and simple to administer treatment option. As deficient and defective cartilage in adults has a limited capacity to heal independently, treatment to aid the process is in high demand. If left untreated, articular cartilage lesions can develop further dysfunction, joint pain and osteoarthritis.
For healthcare professionals and companies, there is a wealth of support and information to help ensure correct and knowledgeable practice. In terms of legal guidelines, there are numerous companies to help with FDA 510k such as http://www.fdathirdpartyreview.com/
Going Forwards
Clinical trials which have taken place so far have suggested that long-term benefits are likely to follow from the treatment of MACI. These trials have supported the efficiency and safety of the procedures. However, some side effects were present, including back pain, headaches and cold-like symptoms.






























No Comments
Leave a comment Cancel